- CONTACT US
- AFS
- Business
- Bussiness
- Car
- Career
- Celebrity
- Digital Products
- Education
- Entertainment
- Fashion
- Film
- Food
- Fun
- Games
- General Health
- Health
- Health Awareness
- Healthy
- Healthy Lifestyle
- History Facts
- Household Appliances
- Internet
- Investment
- Law
- Lifestyle
- Loans&Mortgages
- Luxury Life Style
- movie
- Music
- Nature
- News
- Opinion
- Pet
- Plant
- Politics
- Recommends
- Science
- Self-care
- services
- Smart Phone
- Sports
- Style
- Technology
- tire
- Travel
- US
- World
- エンタメ
- スポーツ
- 科学
- 経済

By Michael Erman
NEW YORK (Reuters) -The U.S. Food and Drug Administration can approve new personalized treatments for rare and deadly genetic diseases based on data from a handful of patients, two of the agency's top officials said on Wednesday.
FDA Commissioner Marty Makary and Chief Medical and Scientific Officer Vinay Prasad said in an essay published in the New England Journal of Medicine that for certain conditions, companies could rely on appropriately designed studies with small sample sizes rather than randomized trials. They will rely on biological plausibility and clinical improvements in those early patients.
"Current regulations are onerous and unnecessarily demanding," Makary and Prasad wrote. "For patients and families, there is no time to wait."
The new "plausible-mechanism" pathway would allow the agency to grant marketing authorization after manufacturers demonstrate success with several consecutive patients.
Companies that receive these approvals will be required to collect real-world evidence to confirm efficacy continues and to look for safety issues that might arise.
The new approach will prioritize treatments for rare diseases that are fatal or cause severe childhood disability. Common diseases with unmet medical needs may also qualify.
While makers of cell and gene therapies are likely to be significant beneficiaries of the new approval process, Makary and Prasad said that other types of treatments could also receive licensure this way.
"The FDA will work as a partner and guide in ushering these therapies to market," the officials wrote.
(Reporting by Michael ErmanEditing by Bill Berkrot)
LATEST POSTS
- 1
 Gaza humanitarian efforts reach key milestone as UNICEF vaccinates some 13,000 children
Gaza humanitarian efforts reach key milestone as UNICEF vaccinates some 13,000 children - 2
 IDF uncovers 7 km.-long Gaza terror tunnel where Hamas held Hadar Goldin
IDF uncovers 7 km.-long Gaza terror tunnel where Hamas held Hadar Goldin - 3
 CDC clarifies stance on vaccines and autism, stating no evidence supports the link
CDC clarifies stance on vaccines and autism, stating no evidence supports the link - 4
 Hamas Navy head, engineer of Khan Yunis tunnel network killed in Gaza, IDF confirms
Hamas Navy head, engineer of Khan Yunis tunnel network killed in Gaza, IDF confirms - 5
 Rights groups condemn Israel Police decision to ban Sudan Genocide protests nationwide
Rights groups condemn Israel Police decision to ban Sudan Genocide protests nationwide
 Getting through a Lifelong Change: Individual Examples of overcoming adversity
Getting through a Lifelong Change: Individual Examples of overcoming adversity The Way to Fruitful Weight reduction: Individual Wellbeing Excursions
The Way to Fruitful Weight reduction: Individual Wellbeing Excursions All that You Want to Be familiar with Dental Inserts Centers
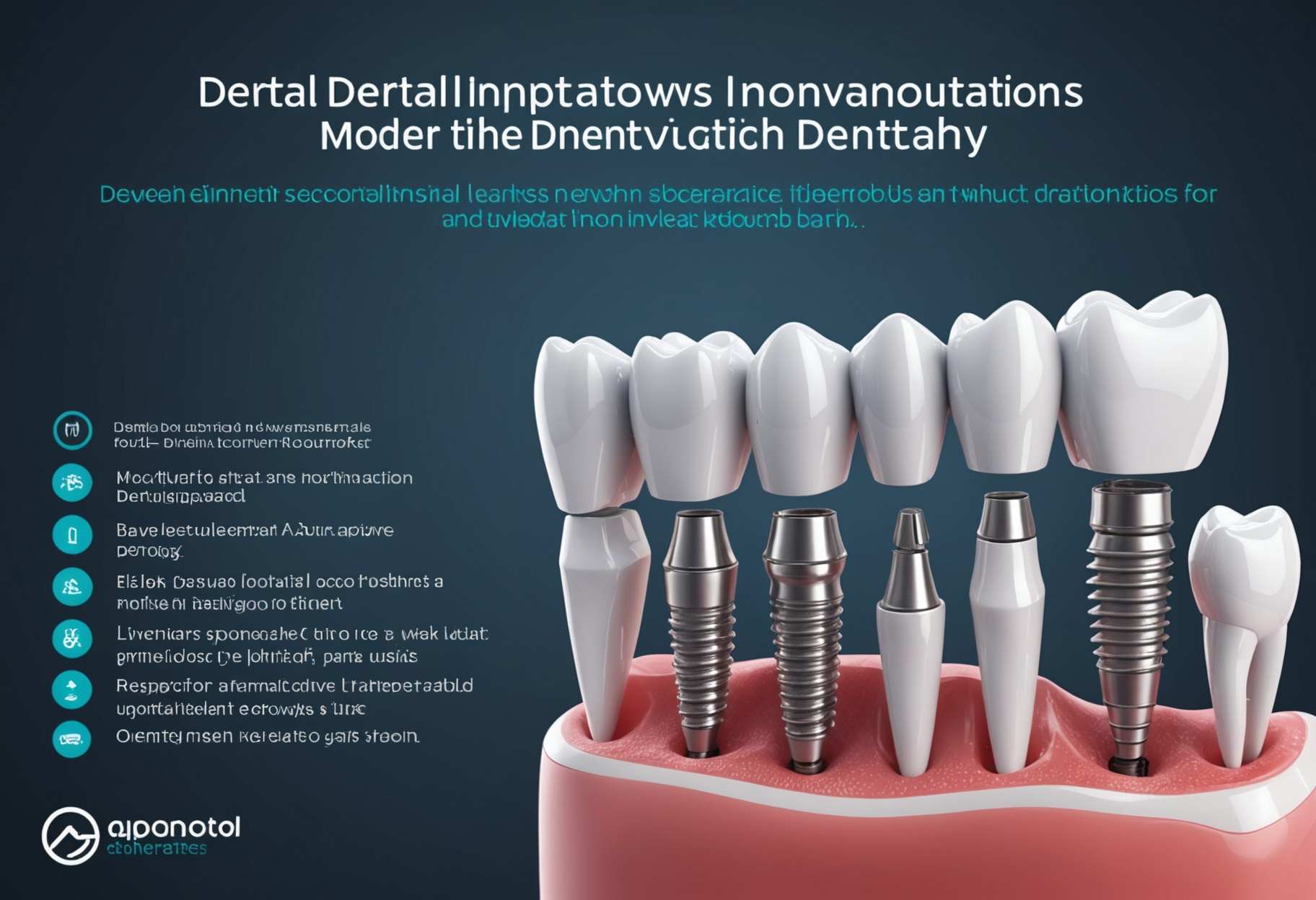
All that You Want to Be familiar with Dental Inserts Centers Dental Embed Developments: Upsetting Current Dentistry
Dental Embed Developments: Upsetting Current Dentistry 5 Wellbeing Applications Assist You With remaining Fit
5 Wellbeing Applications Assist You With remaining Fit PHOTO ESSAY: Summer camp for kids with autoimmune diseases
PHOTO ESSAY: Summer camp for kids with autoimmune diseases Remain Fit and Solid with These Exercise Basics
Remain Fit and Solid with These Exercise Basics ‘The White Lotus’ sparked online interest in risky anxiety pills, study says
‘The White Lotus’ sparked online interest in risky anxiety pills, study says EU health regulator urges immediate vaccinations amid early surge in flu cases
EU health regulator urges immediate vaccinations amid early surge in flu cases